Scientists Mapped the Secret World of Platinum Atoms – And It Changes Everything
Materials science is going to make Earth's resources stretch further, reducing pollution and conflict
NTKP Note:
Catalyst: A catalyst is a substance that speeds up a chemical reaction without being consumed or changed in the process. It helps reactions occur more efficiently by lowering the energy needed for the reaction to take place. - US Dept of Energy
Catalytic converter: E.g. Platinum is used in the production of catalytic converters. “A catalytic converter is an exhaust emission control device in vehicles that reduces harmful pollutants from engine exhaust gases by converting them into less toxic substances, such as carbon dioxide and water vapor. It plays a crucial role in helping vehicles meet environmental regulations and improve air quality.”
BY OLIVER MORSCH, ETH ZURICH, published SciTech JULY 7, 2025
Facebook Twitter Pinterest Telegram
SHARE
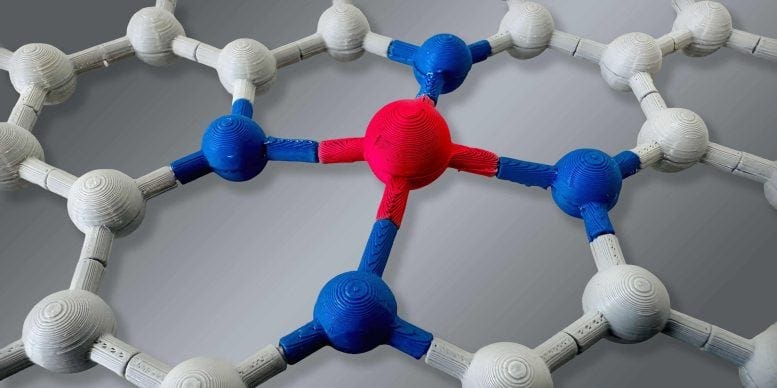
Scientists at ETH Zurich have developed a powerful method to look deep inside single-atom catalysts—materials where every atom plays a vital role in driving chemical reactions. By using a technique called nuclear magnetic resonance (similar to the technology behind MRI scans), they’ve uncovered how platinum atoms interact with their surroundings on an atomic level. This breakthrough could lead to greener, more efficient ways to make chemicals using fewer precious materials.
Researchers used nuclear magnetic resonance to study individual platinum atoms inside solid materials.
The technique revealed which atoms are connected to platinum and how they are positioned in space.
This detailed atomic insight allows scientists to design more uniform and effective single-atom catalysts.
The findings open the door to faster, cleaner chemical reactions that use less platinum and generate less waste.
The method could help advance sustainable chemistry by improving how catalysts are made and used.
The Power and Cost of Catalysis
Catalysis, the process of speeding up chemical reactions by adding a special substance, plays a huge role in modern life. In fact, about 80 percent of all chemical products—from fuels to pharmaceuticals—are made using catalysts. Technologies like car exhaust systems and fuel cells also rely on them.
One of the most effective and widely used catalysts is platinum. It’s incredibly good at triggering reactions, but it comes with major downsides. Platinum is rare, expensive, and its production releases a significant amount of carbon dioxide. That’s why scientists are eager to find ways to use less of it, while still getting maximum performance.
Precision Engineering With Single Atoms
In recent years, researchers have been exploring a new class of catalysts known as single-atom catalysts. These are materials where individual platinum atoms are spread out on a porous surface, such as carbon that’s been infused with nitrogen. The nitrogen atoms help anchor each platinum atom in place, ensuring every single atom plays a role in the chemical reaction.
Now, a team of scientists led by Javier Pérez-Ramírez and Christophe Copéret at ETH Zurich, along with collaborators from the Universities of Lyon and Aarhus, have made an exciting discovery. They found that these single-atom catalysts are much more complex than previously believed.
Using a powerful technique called nuclear magnetic resonance (similar to what’s used in MRI scans), the team revealed that each platinum atom sits in a slightly different environment. These tiny differences in atomic surroundings actually affect how well each atom performs as a catalyst.
Their findings, recently published in Nature, open the door to creating smarter, more efficient catalysts. By fine-tuning the atomic structure around each platinum atom, future materials could deliver better performance with less environmental impact—a big win for both industry and the planet.

From Serendipity to Scientific Innovation
“Until now individual platinum atoms could only be observed through the ‘lens’ of an electron microscope – which looks impressive but doesn’t tell us much about their catalytic properties,” says Pérez-Ramírez. Together with Copéret he thought about how one might characterize the individual platinum atoms more precisely. The collaboration began with a chance encounter during a meeting in the framework of the NCCR Catalysis program.
After the meeting, the two researchers developed the idea to try nuclear magnetic resonance. This method, on which the MRI in a hospital is based and which is typically used for investigating molecules in laboratories, the spins of atomic nuclei in a strong static magnetic field react to oscillating magnetic fields of a certain resonant frequency. In molecules, this resonant frequency depends on how the different atoms are arranged inside the molecule. “Likewise, the resonant frequencies of the single platinum atoms are influenced by their atomic neighbours – for instance, carbon, nitrogen or oxygen – and their orientation relative to the static magnetic field,” Copéret explains.
This leads to many different resonant frequencies, much like the different tones in an orchestra. Finding out which instrument is producing a particular tone isn’t easy. “As luck would have it, during a visit to Lyon one of us met a simulation expert from Aarhus who was visiting there at the same time,” says Copéret. Such encounters, and the collaborations resulting from them, are essential for scientific progress, he adds. Together with the ETH-collaborator, the simulation expert developed a computer code that made it possible to filter out the many different “tones” of the individual platinum atoms from the muddle.
Charting Atomic Neighborhoods
Ultimately, this led to a breakthrough in the description of single-atom catalysts: the research team were now able to compile a kind of map showing the type and position of atoms surrounding the platinum atoms. “This analytical method sets a new benchmark in the field,” says Pérez-Ramírez.
With this method, which is broadly accessible, production protocols for single-atom catalysts can be optimized in such a way that all platinum atoms have tailored environments. This is the next challenge for the team. “Our method is also important from an intellectual property standpoint,” says Copéret: “Being able to precisely describe catalysts at the atomic level enables us to protect them through patents.”
Reference: “Coordination environments of Pt single-atom catalysts from NMR signatures” by Jonas Koppe, Alexander V. Yakimov, Domenico Gioffrè, Marc-Eduard Usteri, Thomas Vosegaard, Guido Pintacuda, Anne Lesage, Andrew J. Pell, Sharon Mitchell, Javier Pérez-Ramírez and Christophe Copéret, 4 June 2025, Nature.
DOI: 10.1038/s41586-025-09068-x
Never miss a breakthrough: Join the SciTechDaily newsletter.


